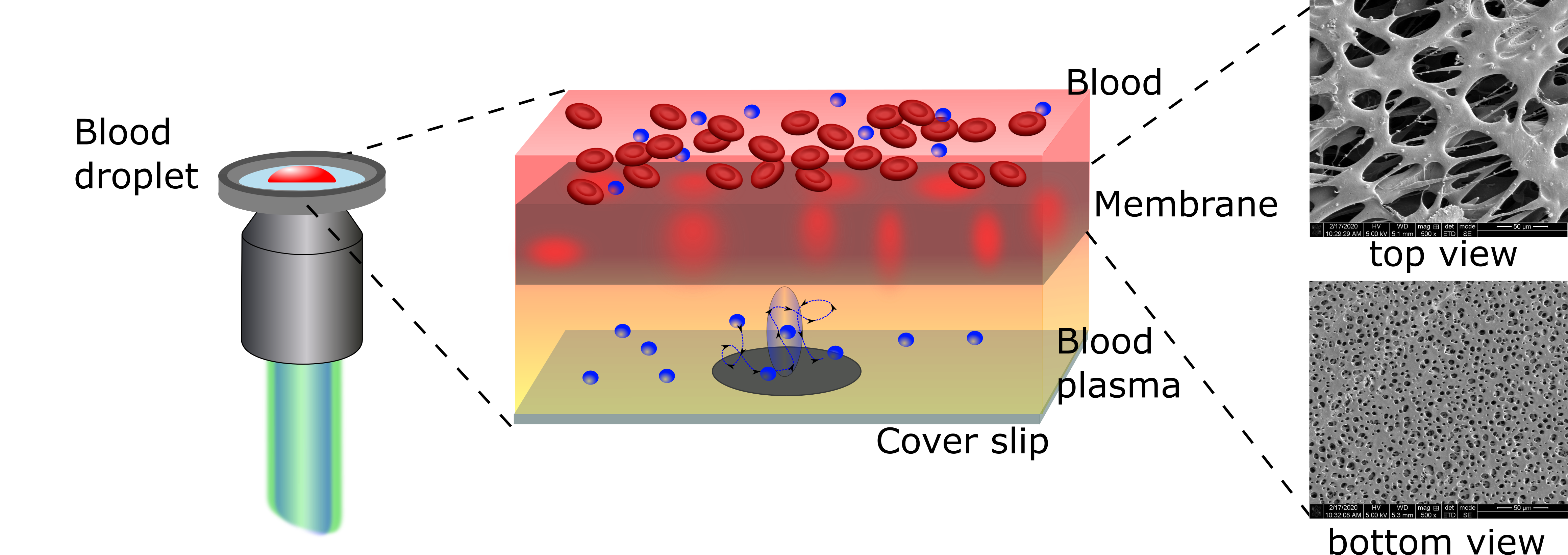
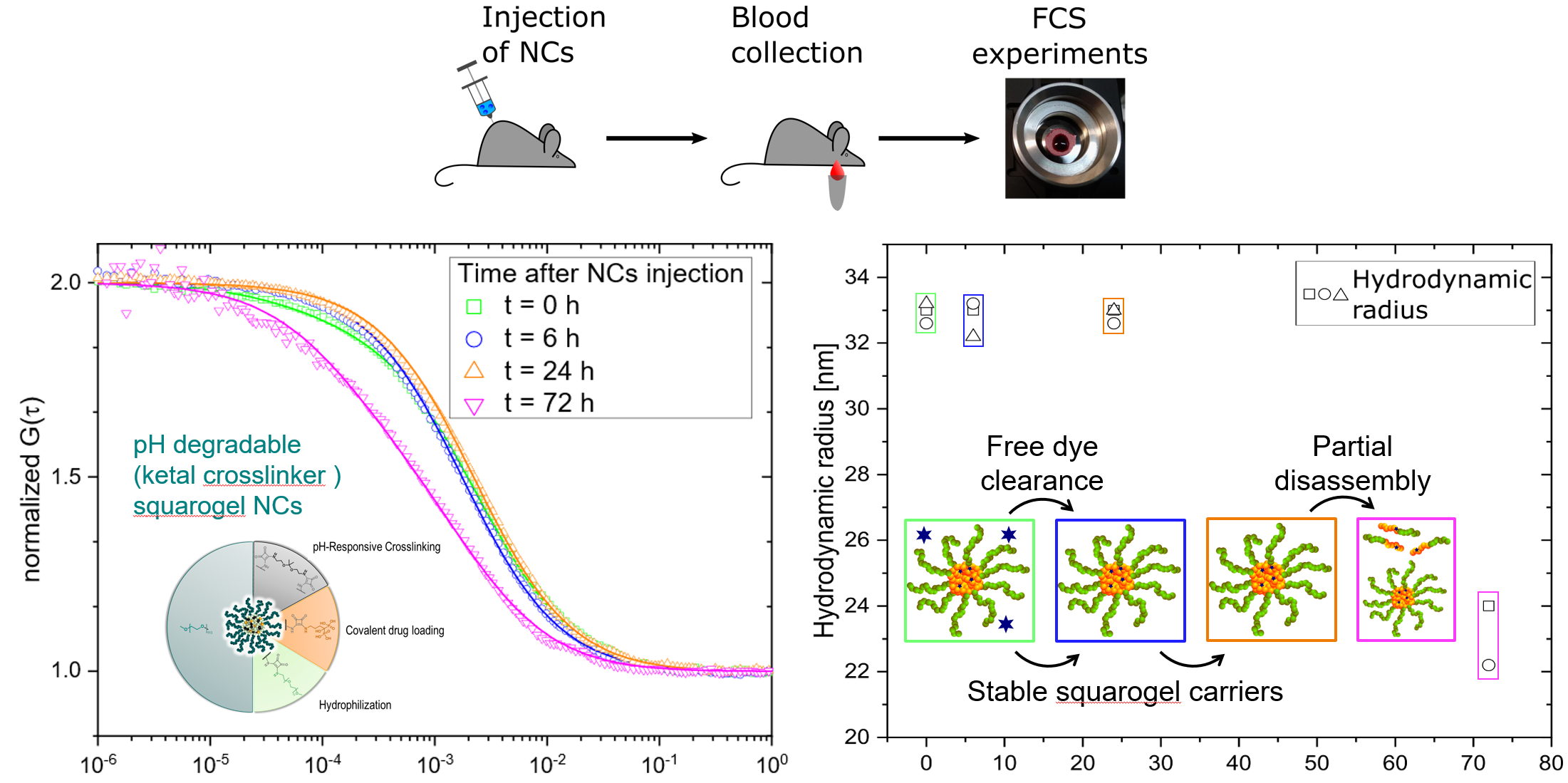
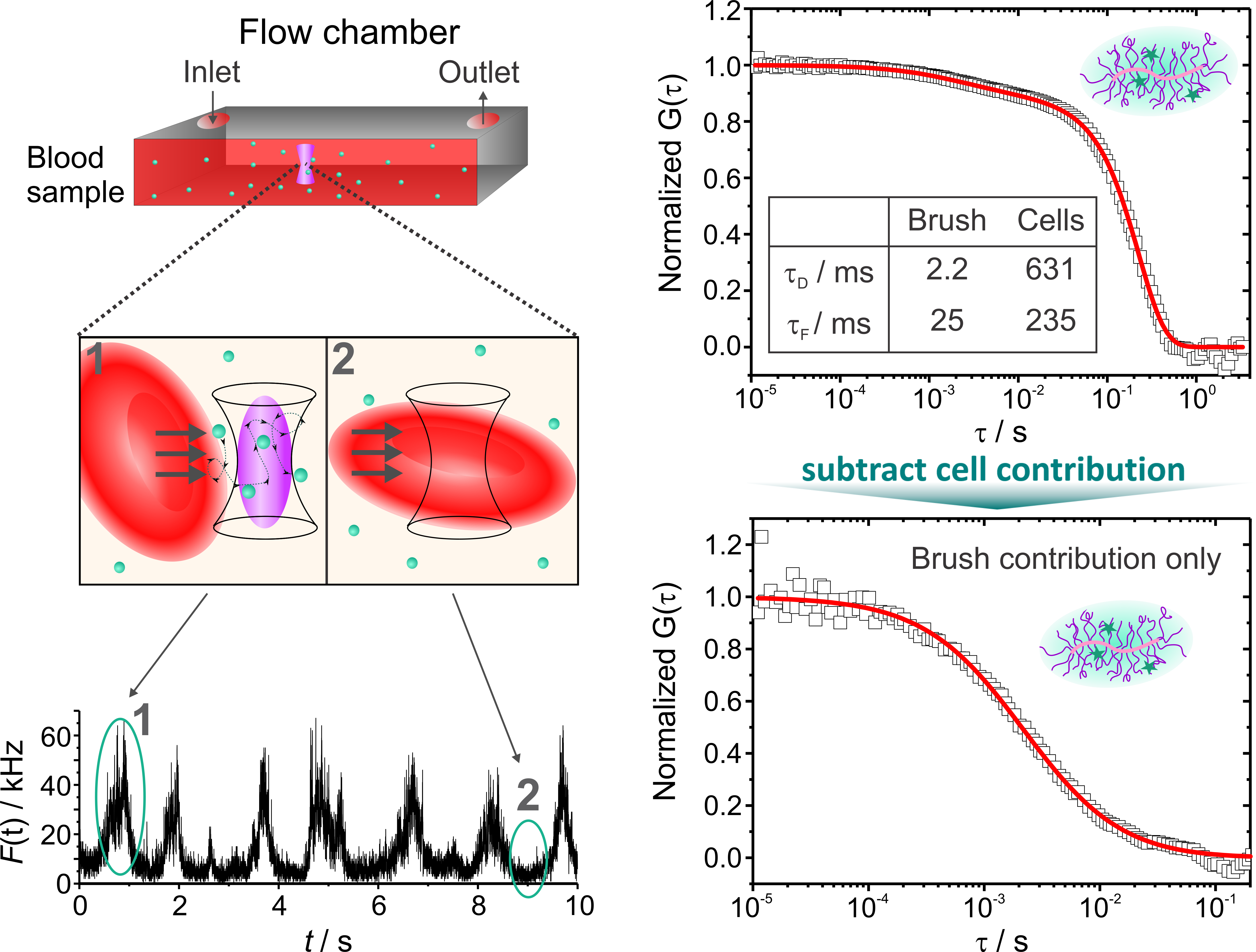
|
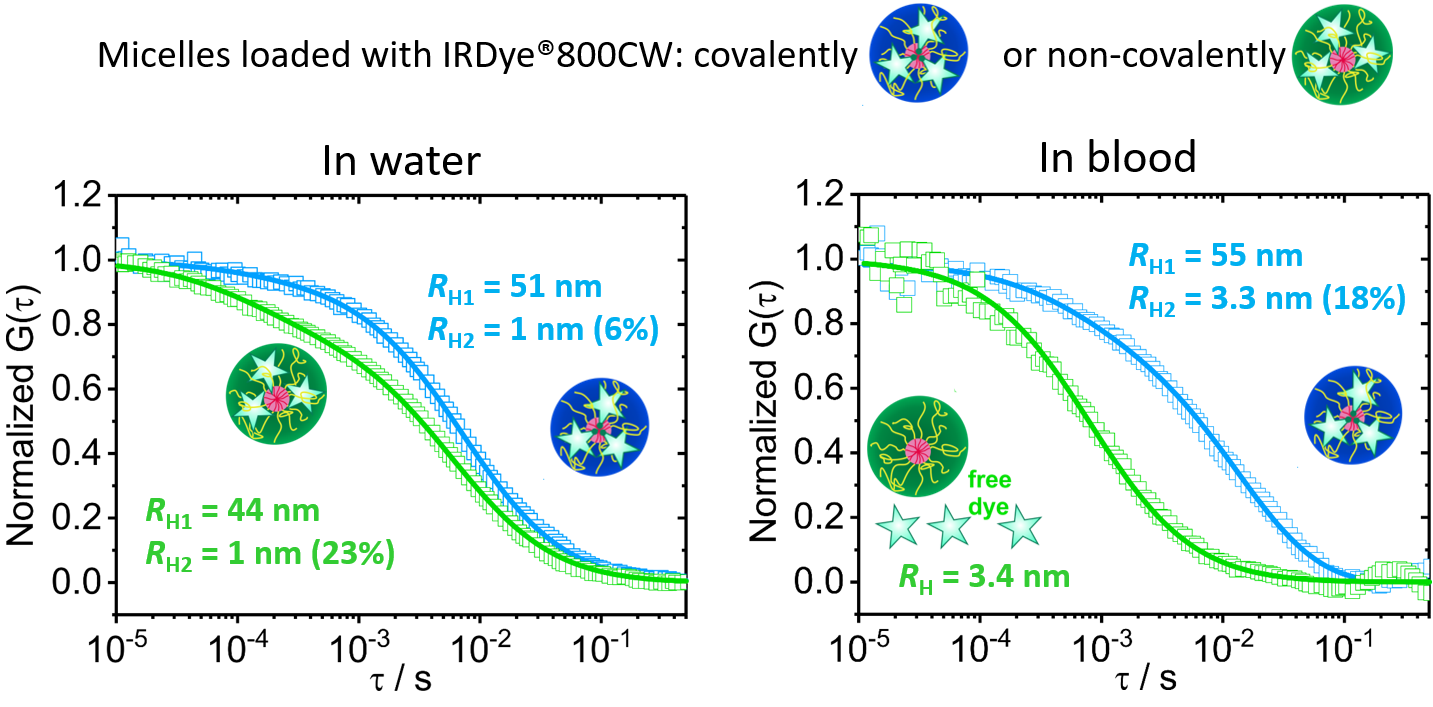
Figure 4. Loading stability of core-crosslinked micelle nanocarriers in blood.
Normalized autocorrelation curves (symbols) and the corresponding fits
(lines) are shown for core-crosslinked micelles that were either
covalently (blue color) or non-covalently (green color) loaded
with IRDye®800CW. In water (left) the dye is mainly loaded in the
core-crosslinked micelles and only a small fraction of free dye was
detected for both systems. In blood (right) the covalently loaded dye
is still in the micelles even after 30 hours incubation, but the non-covalently (hydrophobically) loaded dye is fully released after 30 minutes.
In cooperation with M. Barz (Uni Mainz and MPIP, AK Weil).
- Schmitt S, Nuhn L, Barz M, Butt H-J, Koynov K:
Shining Light on Polymeric Drug Nanocarriers with Fluorescence Correlation Spectroscopy.
Macromolecular Rapid Communications, 2022, 43, 2100892 (doi 10.1002/marc.202100892)
- Schmitt
S, Huppertsberg A, Klefenz A, Kaps L, Mailaender
V, Schuppan D, Butt H-J, Nuhn L, Koynov K:
Fluorescence Correlation Spectroscopy Monitors the Fate of Degradable Nanocarriers in the Blood Stream
Biomacromolecules, 2022, 23, 1065-1074 (doi 10.1021/acs.biomac.1c01407)
-
Negwer I, Best A, Schinnerer M, Schafer O, Capeloa L, Wagner M, Schmidt M, Mailander V, Helm M, Barz M, Butt HJ, Koynov K:
Monitoring drug nanocarriers in human blood by near-infrared fluorescence correlation spectroscopy.
Nature Communications, 2018, 9, 5306 (doi 10.1038/s41467-018-07755-0)
- Czysch C, Medina-Montano C, Zhong Z, Fuchs
A, Stickdorn J, Winterwerber P, Schmitt S, Deswarte
K, Raabe M, Scherger M, Combes F, De Vrieze
J, Kasmi S, Sandners N, Lienenklaus S, Koynov
K, Raeder H-J, Lambrecht B, David S, Bros
M, Schild H, Grabbe S, De Geest B, Nuhn L:
Transient Lymph Node Immune Activation by Hydrolysable Polycarbonate Nanogels
Advanced Functional Materials, 2022, 32, 2203490 (doi 10.1002/adfm.202203490)
- Liu
W, Alam N, Liu Y, Agafonov V, Qi H, Koynov
K, Davydov V, Uzbekov R, Kaiser U, Lasser
T, Jelezko F, Ermakova A, Weil T:
Silicon-Vacancy Nanodiamonds as High Performance Near-Infrared Emitters for Live-Cell Dual-Color Imaging and Thermometry
Nano Letters, 2022, 22, 2881-2888 (doi 10.1021/acs.nanolett.2c00040)
- Doan-Nguyen TP, Jiang S, Koynov K, Landfester K, Crespy D:
Ultrasmall Nanocapsules Obtained by Controlling Ostwald Ripening
Angewandte Chemie-International Edition, 2021, 60, 18094-18102 (doi 10.1002/anie.202103444)
- Bauer TA, Horvat NK, Marques O, Chocarro S, Mertens C, Colucci S,
Schmitt S, Carrella LM, Morsbach S, Koynov K, Fenaroli F, Blumler P,
Jung M, Sotillo R, Hentze MW, Muckenthaler MU, Barz M:
Core Cross-Linked Polymeric Micelles for Specific Iron Delivery: Inducing Sterile Inflammation in Macrophages
Advanced Healthcare Materials, 2021, 10, 2100385 (doi 10.1002/adhm.202100385)
- Van Lysebetten D, Malfanti A, Deswarte K, Koynov K, Golba B, Ye TT,
Zhong ZF, Kasmi S, Lamoot A, Chen Y, Van Herck S, Lambrecht BN, Sanders
NN, Lienenklaus S, David SA, Vicent MJ, De Koker S, De Geest BG:
Lipid-Polyglutamate Nanoparticle Vaccine Platform
Acs Applied Materials & Interfaces, 2021, 13, 6011-6022 (doi 10.1021/acsami.0c20607)
- Lueckerath T, Koynov K, Loescher S, Whitfield C, Nuhn L, Walther A, Barner-Kowollik C, Ng D, Weil Tanja :
DNA-Polymer Nanostructures by RAFT Polymerization and Polymerization-Induced Self-Assembly
Angewandte Chemie - International Edition, 2020, 59, 15474-15479 (doi 10.1002/anie.201916177)
-
Chen C, Wunderlich K, Mukherji D, Koynov K, Heck A, Raabe M, Barz M, Fytas G, Kremer K, Ng DYW, Weil T:
Precision Anisotropic Brush Polymers by Sequence Controlled Chemistry.
J. Am. Chem. Soc. 2020, 142, 1332-1340 (doi 10.1021/jacs.9b10491)
|